
Federal Circuit Limits the Application of Obviousness-Type Double Patenting for Patents in the Same Family
6 min read
Explore Trendscape
Our take on the interconnected global trends that are shaping the business climate for our clients.
On August 13, 2024, a three-judge panel of the Court of Appeals for the Federal Circuit issued a decision, authored by Judge Lourie, in Allergan USA, Inc. v. MSN Laboratories Private Ltd., No. 24-1061, which limits the application of obviousness-type double patenting. The Federal Circuit held that a first-filed, first-issued, later-expiring patent claim cannot be invalidated for obviousness-type double patenting by a later-filed, later-issued, earlier-expiring reference patent claim having a common priority date.
Proceedings below – The District Court Finds Claim Invalid for Obviousness-Type Double Patenting based on Cellect
After Sun Pharmaceutical Industries Limited's ("Sun") submitted an Abbreviated New Drug Application seeking FDA approval to market and sell a generic version of Allergan's drug Viberzi® (eluxadoline tablets), Allergan filed a patent infringement suit against Sun in the District of Delaware asserting several patents covering Viberzi®, including U.S. Patent No. 7,741,356 ("the '356 patent"), the first patent to cover eluxadoline.1
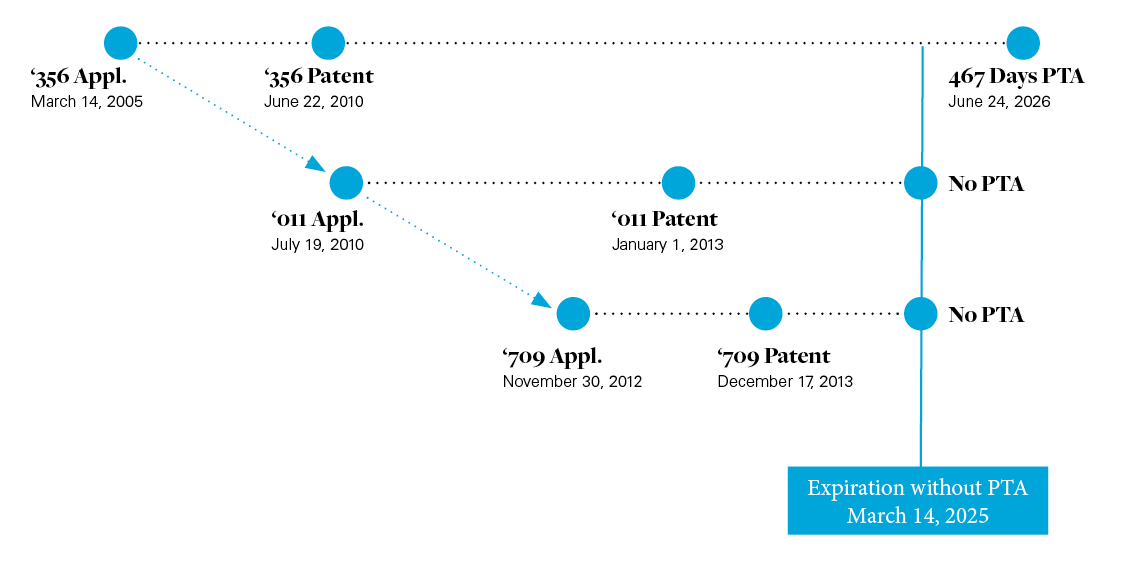
Before the district court, Sun argued that claim 40 of the '356 patent is invalid for obviousness-type double patenting ("ODP") over claim 33 of U.S. Patent No. 8,344,011 ("the '011 patent") and claim 5 of the U.S. Patent No. 8,609,709 ("the '709 patent") patent because the claims are not patentably distinct and because claim 40 expires after the reference claims of the '011 and '709 patents.2 The '011 and '709 patents issued from continuation applications sharing a priority date with the '356 patent.3 The filing, issuance, expiration dates, and patent term adjustment ("PTA") for the three patents is shown below:
In response, Allergan argued that because the '356 patent was the first patent claiming eluxadoline to be filed and the first patent to issue, claim 40 is not subject to ODP over the later-filed, later-issued claims of the reference patents.4 Allergan did not contest that claim 40 is not patentably distinct from the reference claims.5
This case follows last year's Federal Circuit decision in Cellect.6 Cellect involved patents claiming priority from a single application that would have expired on the same day but for PTA.7 During reexamination proceedings, the claims of the since-expired patents were found invalid for ODP because the various awards of PTA had resulted in the patent owner receiving an extension of patent term on patentably indistinct inventions.8 On appeal, the Federal Circuit affirmed, holding that, when it comes to evaluating ODP on a patent that has received PTA, the relevant expiration date is the expiration date, including PTA.9
Relying on Cellect, Judge Andrews agreed with Sun, finding Allergan's "first-filed, first-issued" distinction "immaterial" because "[w]hen analyzing ODP, a court compares patent expiration dates, rather than filing or issuance dates."10 The district court reasoned that Allergan was seeking "a case-by-case review of equitable considerations to determine if a patent owner received an unjust time extension," an analysis it deemed "rejected" by Cellect.11
On Appeal – The Federal Circuit Reverses
Judge Lourie authored the opinion for the Federal Circuit panel that also included Judges Dyk and Reyna. The Court reversed the district court's judgment invalidating claim 40 of the '356 patent for ODP and held that a first-filed, first-issued, later-expiring claim cannot be invalidated by a later-filed, later-issued, earlier-expiring reference claim having a common priority date.12
Rejecting the district court's reliance on Cellect in invalidating claim 40 of the '356 patent, the Federal Circuit noted that "Cellect answered a different question than that at issue here."13 The Court explained that, while Cellect requires that the ODP analysis for the '356 patent must consider the expiration date after PTA is added, "[i]t does not follow, however, that the '356 patent must be invalidated by the '011 and '709 reference patents simply because it expires later."14 According to the Court, Cellect did not address "under what circumstances can a claim properly serve as an ODP reference."15
Addressing the instant case, the Federal Circuit held that the claims of the '011 and '709 reference patents are not proper ODP references that can be used to invalidate claim 40 of the '356 patent.16 The Court reasoned that this conclusion was consistent with "the purpose of the ODP doctrine, which is to prevent patentees from obtaining a second patent on a patentably indistinct invention to effectively extend the life of a first patent to that subject matter."17 Since the applications leading to the '011 and '709 patent were filed after the '356 patent issued, "each of the '011 and '709 patents is unquestionably ‘second' to that patent."18
The Court distinguished Gilead, which considered ODP for patents with different priority and expiration dates that claimed patentably indistinct subject matter.19 Under those circumstances, the Federal Circuit held that a later-issued but earlier-expiring patent can qualify as a ODP reference to invalidate an earlier-issued but later-expiring patent.20 The Court pointed out that Gilead "did not address the role of filing dates" and that the holding in Gilead "was expressly limited to the circumstances of that case."21 Moreover, the Court explained that, unlike the challenged patent in Gilead, the '356 patent was the first patent in its family to be filed and to issue, so it did not extend any period of exclusivity on the claimed subject matter.22
The Court recognized that prosecution of a "first-of-its-kind invention" is more likely to result in PTA than prosecution of a later filed continuing application covering a modification of that invention.23 Accordingly, the later-filed, later-issued child patent expires first.24 However, the Court noted, the child patent does not "result in any extension of patent term of the invention claimed in the parent patent given that it expires first."25 And the parent patent cannot be said to extend the term of the invention claimed in the child patent when "the claims in the child patent did not even exist until after the parent patent issued."26
The Court concluded that holding that a first-filed, first-issued parent patent with PTA can be invalidated by a later-filed, later-issued child patent "would not only run afoul of the fundamental purposes of ODP, but effectively abrogate the benefit Congress intended to bestow on patentees when codifying PTA."27
More Limited ODP Attacks?
The Cellect decision appeared to leave little room to defend against ODP attacks for patents whose expiration dates were extended solely because of PTA. Therefore, patent owners will welcome the Federal Circuit's decision, even if the holding in this case only applies patents that issued before a continuation application in the same family was filed. Because the Federal Circuit's analysis relied heavily on the purpose of the ODP doctrine as preventing unjustified extension of patent term, patent owners might argue in future cases that other patents should be insulated from ODP challenges under this reasoning.
1 Allergan USA, Inc. v. MSN Laboratories Private Ltd., Case No. 24-1061, __ F.4th __ (Fed. Cir. Aug. 13, 2024), slip op. at 7-8.
2 Id. at 9.
3 Id. at 4-5.
4 Id. at 9.
5 Id.
6 Id. at 14 (citing In re Cellect, LLC, 81 F.4th 1216 (Fed. Cir. 2023)).
7 Id. (citation omitted).
8 Id. (citation omitted).
9 Id. (citation omitted).
10 Id. at 9 (citation omitted).
11 Id. (citation omitted).
12 Id. at 11.
13 Id. at 16.
14 Id.
15 Id.
16 Id.
17 Id. (emphasis in the original).
18 Id. at 18 (citing Gilead Scis., Inc. v. Natco Pharma Ltd., 753 F.3d 1208, 1215-17 (Fed. Cir. 2014).
19 Id. (citation omitted).
20 Id. (citation omitted).
21 Id. (citation omitted).
22 Id. at 19.
23 Id. at 19-20.
24 Id. at 20.
25 Id.
26 Id.
27 Id.
White & Case means the international legal practice comprising White & Case LLP, a New York State registered limited liability partnership, White & Case LLP, a limited liability partnership incorporated under English law and all other affiliated partnerships, companies and entities.
This article is prepared for the general information of interested persons. It is not, and does not attempt to be, comprehensive in nature. Due to the general nature of its content, it should not be regarded as legal advice.
© 2024 White & Case LLP

 View full image here
View full image here

